Page 30 - tmp
P. 30
137
Desorption of Cs from Brachythecium mildeanum moss using solutions that imitate
acid rain pH 4.60-6.50
Danica Anđelić
Regional Center for talented youth Belgrade II, Serbia, da.andjelic04@gmail.com
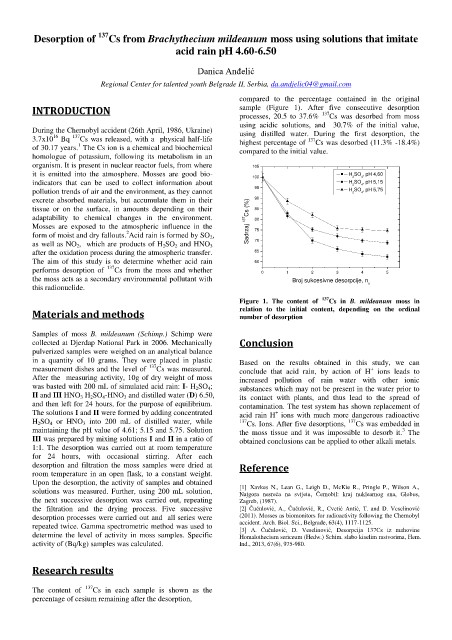
compared to the percentage contained in the original
INTRODUCTION sample (Figure 1). After five consecutive desorption
processes, 20.5 to 37.6% 137 Cs was desorbed from moss
using acidic solutions, and 30.7% of the initial value,
During the Chernobyl accident (26th April, 1986, Ukraine) using distilled water. During the first desorption, the
16
3.7x10 Bq 137 Cs was released, with a physical half-life highest percentage of 137 Cs was desorbed (11.3% -18.4%)
1
of 30.17 years. The Cs ion is a chemical and biochemical compared to the initial value.
homologue of potassium, following its metabolism in an
organism. It is present in nuclear reactor fuels, from where 105
it is emitted into the atmosphere. Mosses are good bio- 100 H SO , pH 4,60
4
2
indicators that can be used to collect information about H SO , pH 5,15
4
2
pollution trends of air and the environment, as they cannot 95 H SO , pH 5,75
4
2
excrete absorbed materials, but accumulate them in their 90
tissue or on the surface, in amounts depending on their 85
adaptability to chemical changes in the environment. 80
Mosses are exposed to the atmospheric influence in the Sadrzaj 137 Cs (%) 75
2
form of moist and dry fallouts. Acid rain is formed by SO 2 ,
as well as NO 2 , which are products of H 2 SO 2 and HNO 3 70
after the oxidation process during the atmospheric transfer. 65
The aim of this study is to determine whether acid rain 60
performs desorption of 137 Cs from the moss and whether 0 1 2 3 4 5
the moss acts as a secondary environmental pollutant with Broj sukcesivne desorpcije, n
this radionuclide. x
Figure 1. The content of 137 Cs in B. mildeanum moss in
Materials and methods relation to the initial content, depending on the ordinal
number of desorption
Samples of moss B. mildeanum (Schimp.) Schimp were
collected at Djerdap National Park in 2006. Mechanically Conclusion
pulverized samples were weighed on an analytical balance
in a quantity of 10 grams. They were placed in plastic Based on the results obtained in this study, we can
measurement dishes and the level of 137 Cs was measured. conclude that acid rain, by action of H ions leads to
+
After the measuring activity, 10g of dry weight of moss increased pollution of rain water with other ionic
was basted with 200 mL of simulated acid rain: I- H 2 SO 4 ; substances which may not be present in the water prior to
II and III HNO 3 H 2 SO 4 -HNO 3 and distilled water (D) 6.50, its contact with plants, and thus lead to the spread of
and then left for 24 hours, for the purpose of equilibrium. contamination. The test system has shown replacement of
The solutions I and II were formed by adding concentrated acid rain H ions with much more dangerous radioactive
+
H 2 SO 4 or HNO 3 into 200 mL of distilled water, while 137 Cs. Ions. After five desorptions, 137 Cs was embedded in
maintaining the pH value of 4.61; 5.15 and 5.75. Solution the moss tissue and it was impossible to desorb it. The
3
III was prepared by mixing solutions I and II in a ratio of obtained conclusions can be applied to other alkali metals.
1:1. The desorption was carried out at room temperature
for 24 hours, with occasional stirring. After each
desorption and filtration the moss samples were dried at Reference
room temperature in an open flask, to a constant weight.
Upon the desorption, the activity of samples and obtained
solutions was measured. Further, using 200 mL solution, Xavkes N., Lean G., Leigh D., McKie R., Pringle P., Wilson A.,
Najgora nesreća na svijetu, Černobil: kraj nuklearnog sna, Globus,
the next successive desorption was carried out, repeating Zagreb, (1987).
the filtration and the drying process. Five successive 2 Čučulović, A., Čučulović, R., Cvetić Antić, T. and D. Veselinović
desorption processes were carried out and all series were (2011). Mosses as biomonitors for radioactivity following the Chernobyl
repeated twice. Gamma spectrometric method was used to accident. Arch. Biol. Sci., Belgrade, 63(4), 1117-1125.
determine the level of activity in moss samples. Specific 3 A. Čučulović, D. Veselinović, Desorpcija 137Cs iz mahovine
Homalothecium sericeum (Hedw.) Schim. slabo kiselim rastvorima, Hem.
activity of (Bq/kg) samples was calculated. Ind., 2013, 67(6), 975-980.
Research results
The content of 137 Cs in each sample is shown as the
percentage of cesium remaining after the desorption,